INTRODUCTION
In recent years, many industries have been actively working towards reducing their detection limits often because of regulatory requirements. Another example is evident in the recent FDA proposal of a “close to zero” plan to eradicate trace metal contaminants from baby food1.
Reducing detection limits presents some challenges and laboratories need to have specific protocols and the right set-up to control possible contamination and ensure a robust approach.
Any sample preparation for elemental analysis requires the addition of reagents and the handling of these reagents, even if ultrapure, is a key factor in achieving lower detection limits and ensuring reliable quantification limits. Certainly, the quality of the acid plays a key role, but if not combined with proper handling, the reagent can easily be contaminated, affecting the blank values. Apart from reagent contamination, other sources of contamination include digestion vessels that have not been sufficiently cleaned between runs and dirty glassware.
When working at trace level analysis, the choice falls on ultrapure reagents including acids and water. What is not often considered is the potential for the presence of the analyst to contaminate the reagents in routine operations that could lead to inconsistent results. In fact, human skin, hair and sweat contain contaminants such as Zn, Cd, Pb, Fe, Cu, Ni, Mn, and Na. In addition, the use of cosmetics and the presence of watches, rings, bracelets, and other jewellery further increases the risk of contamination during handling.
For example, Rodushkin et al.2 reported increases in levels of 30 to 50 times for Bi and Sb probably originating from make-up worn by the analyst where these are used as additives for bacterial growth inhibition in some formulations or found in one of the components of the black pigment in mascara. The same publication also highlights the routine use of pipettes as a source of contamination when working at trace levels, either due to the presence of metal components in the pipettes or simply due to contamination on the tips. Even leaving a bottle of ultrapure reagent uncapped in the laboratory will get contaminated during regular operations.
This article will explore how the implementation of an automated dosing system can
effectively overcome the challenges of reagent contamination and also investigate the critical task of adding concentrated acid and other reagents into digestion vessels, a process that has traditionally relied on manual methods like pipettes. This crucial step faces several limitations in addition to contamination:
– Safety Concerns
– Time consuming operation
METHODS

Figure1: Auto dispensing easyFILL system
The Milestone easyFILL from Analytix used in this study is an automated dosing station specifically design to be integrated into the sample preparation process (Figure 1). It controls direct addition of reagents in most digestion vessels and vials without exposing the operator to the reagents.

Figure 2: Graphical interface for simplified procedures
To enable easy operation the system is controlled via a dedicated graphical interface (Figure 2). This allows new users to quickly understand the process of control and can simply select the type and volume of reagents and then the system begins the addition. For routine operations, the user has only to select a customised method and press “Start” to begin the addition.
The system is equipped with six lines for different reagents that through a peristaltic pump are directly loaded into digestion vessels and vials. The six lines can be a combination of solvents and water or acids and water however this study looks at the most common reagents used in food testing laboratories namely ultrapure water, trace metal grade nitric and hydrochloric acid, and hydrogen peroxide.
Two different procedures for adding reagents to vessels were compared: the conventional method using a bottle-top dispenser (manual addition) and an automated method using easyFILL. The analysis was performed with ICP-MS TQ on 67 elements representing both the major and trace elements typically analysed in food matrices. The easyFILL system had been in regular use for 12 months prior to the study.
RESULTS
Table 1 shows the results for levels of major elements for manual dispensing with a bottle top system compared to auto dispensing using easyFILL. The levels for the auto dispensing method are significantly lower compared to the manual method. As indicated by the colour codes there were a few sporadic values where the easyFILL bias was higher however these are not significantly different and overall, the auto dispensing method shows favourable results.
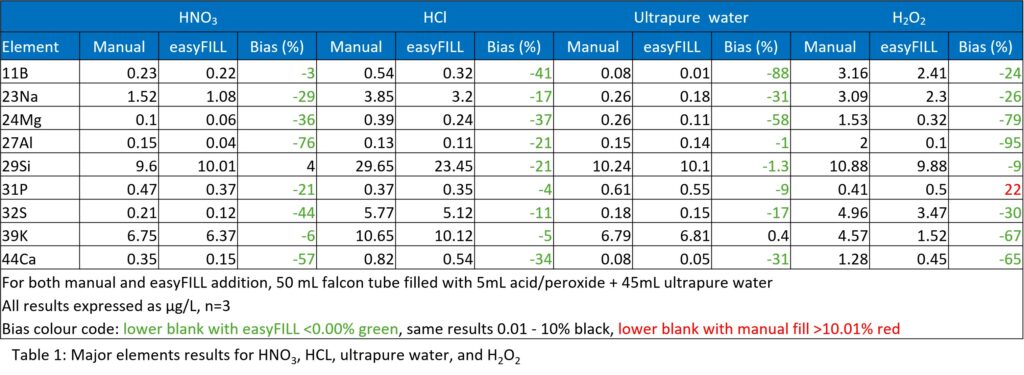
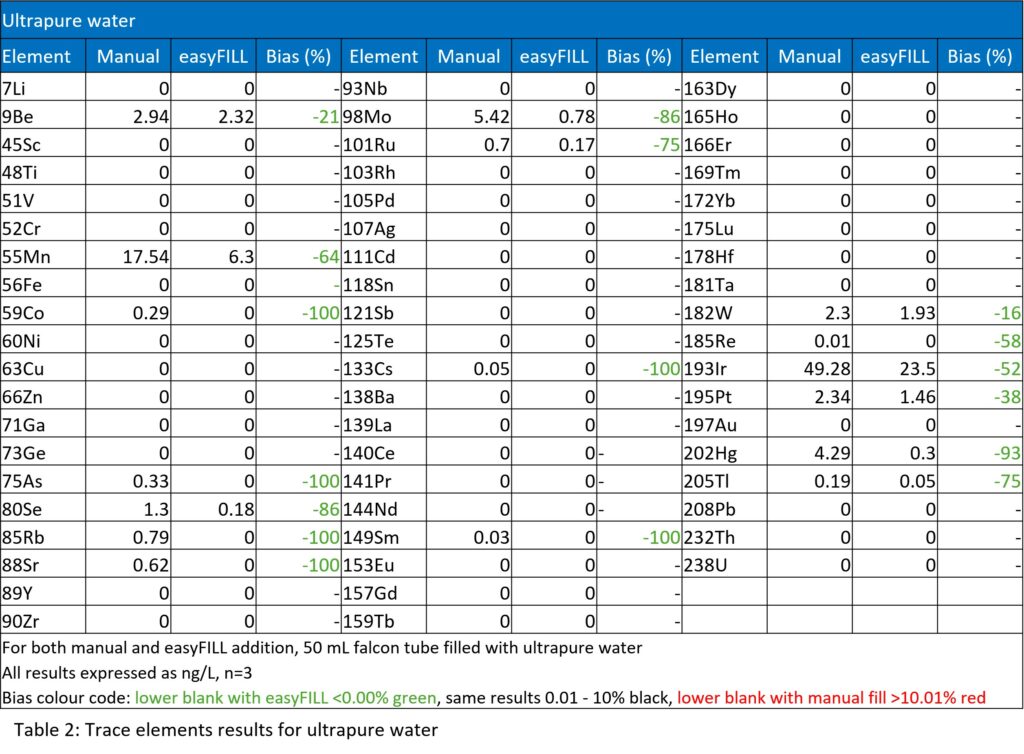
Table 2 shows the results for levels of trace elements in ultrapure water for manual dispensing with a bottle top system compared to auto dispensing using easyFILL. Although for this set of elements most levels were the same, where there where differences, the auto dispensing method results were favourable. Results for other acids and peroxide are available on request and again show that the auto dispensing method produces significantly lower levels.
CONCLUSION
The auto dispensing easyFILL system demonstrated better acid dispensing capability and a superior analytical blank value compared to the manual bottle top dispensing method by removing analyst contamination and human error. The lower levels achieved by easyFILL for both the major and trace elements can be explained by the use of metal-free lines, the use of sealed bottles so there is no risk of the reported contamination from leaving them open during normal operations, and the removal of other potential sources of contamination associated with reagent handling.
Coupled with consideration of the safety aspects of the elimination of acid handling, the easyFILL is a true asset to any laboratory performing trace element analysis.
Request further information
REFERENCES
- FDA, The Baby Food Safety Act of 2021
- I. Rodushkin, E. Engström, D. C. Baxter Anal. Bioanal. Chem. 396 (2010) 365-377.
